Impressive Info About How To Choose Indicator For Titration
![18.3 Identify An Appropriate Indicator For A Titration [Hl Ib Chemistry] - Youtube](https://s3-us-west-2.amazonaws.com/courses-images-archive-read-only/wp-content/uploads/sites/887/2015/05/23213915/CNX_Chem_14_07_indicators.jpg)
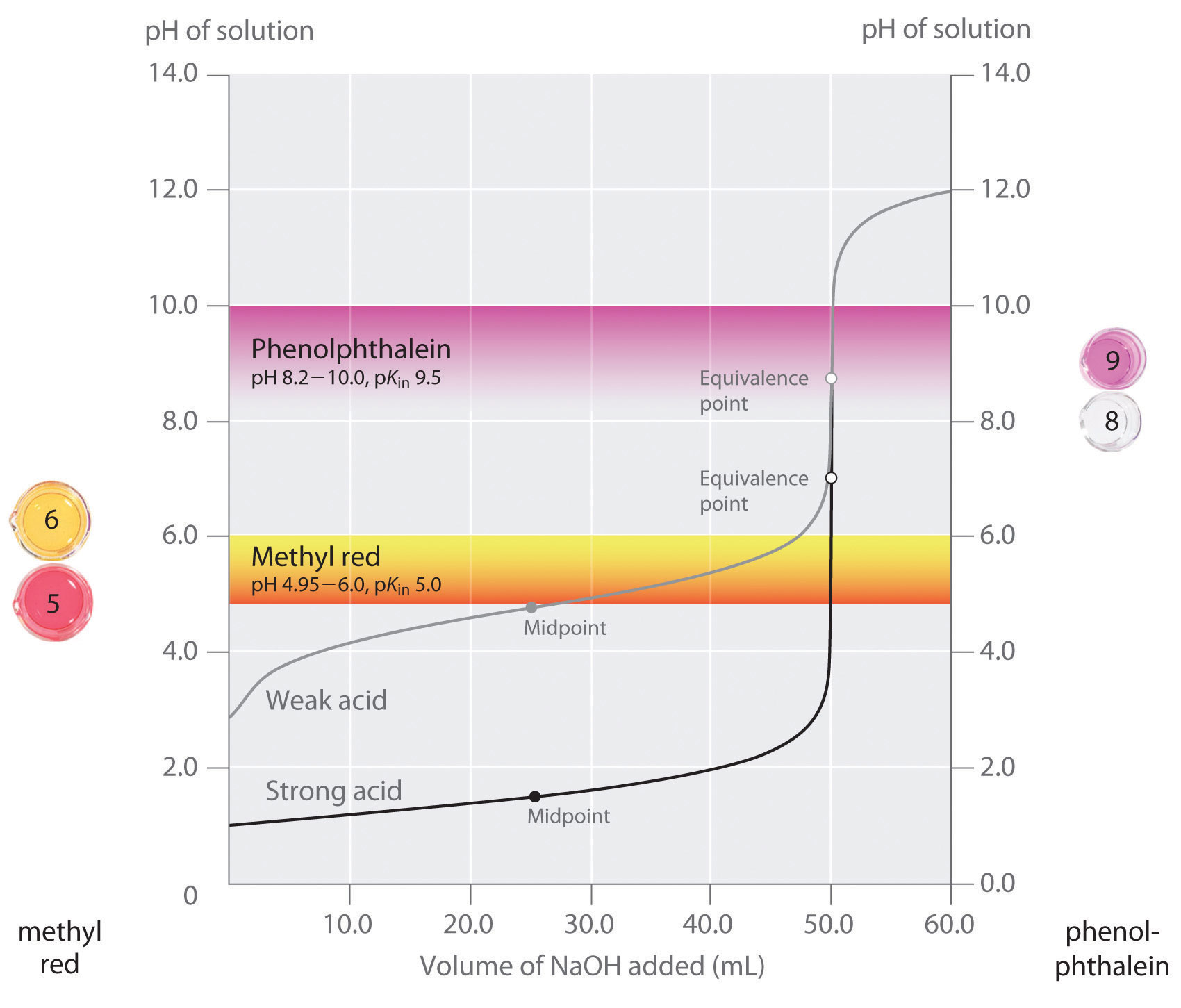
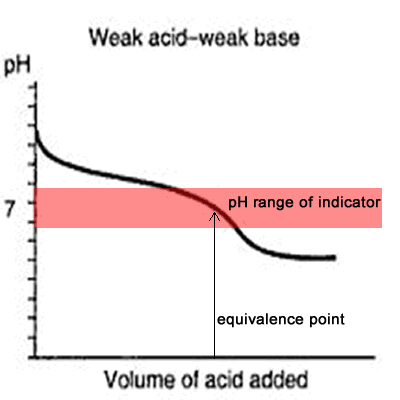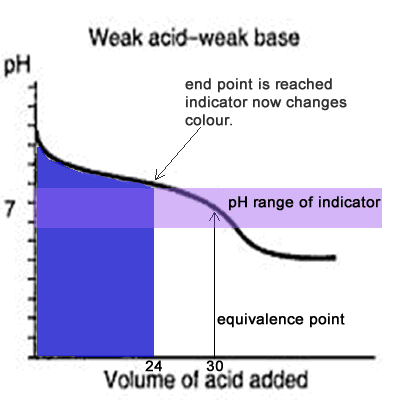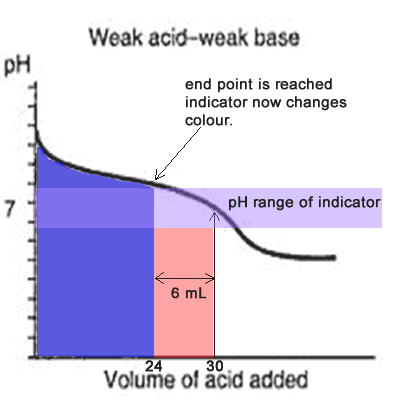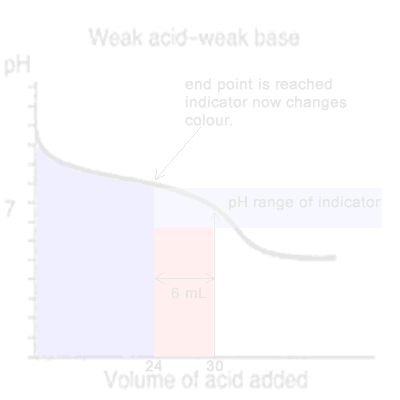
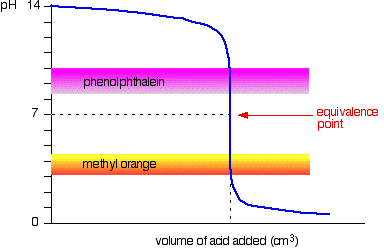
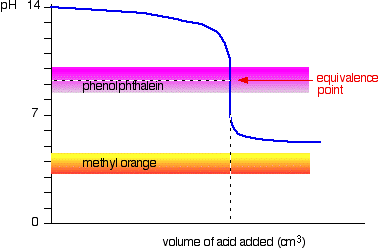
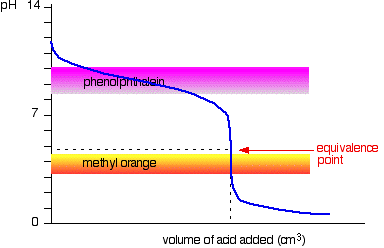
You see a color change in this range.
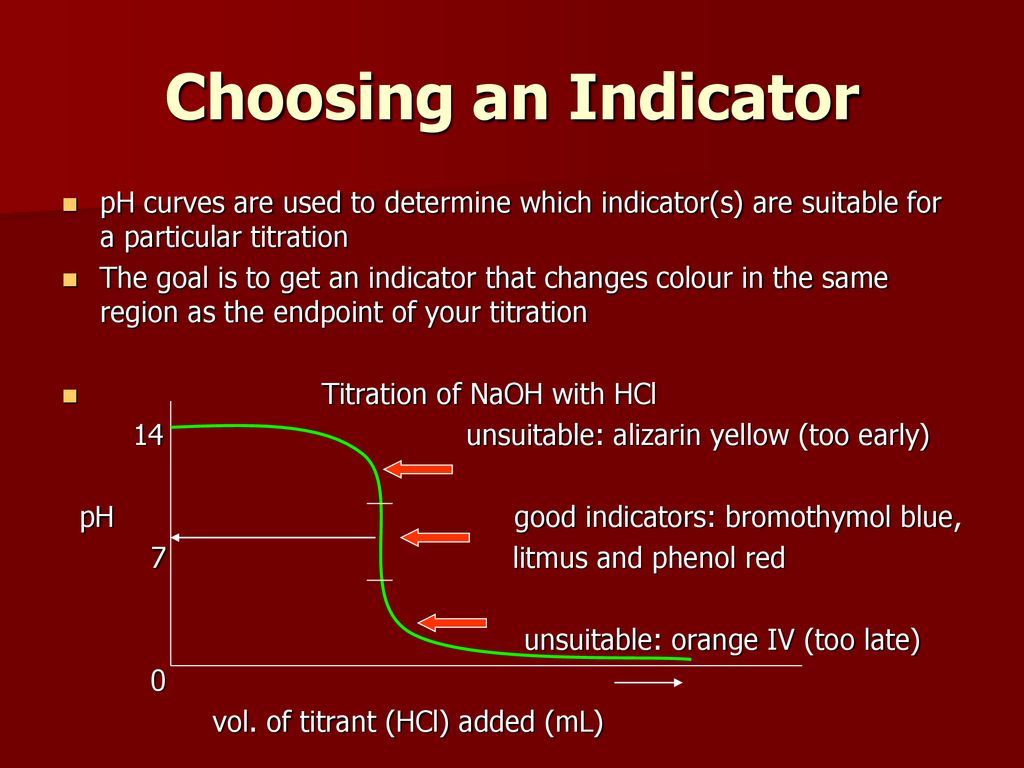
How to choose indicator for titration. Choosing the best indicator for different titrations depending on the ph at the equivalence point. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. This video explains how to choose the best indicator for titration reaction
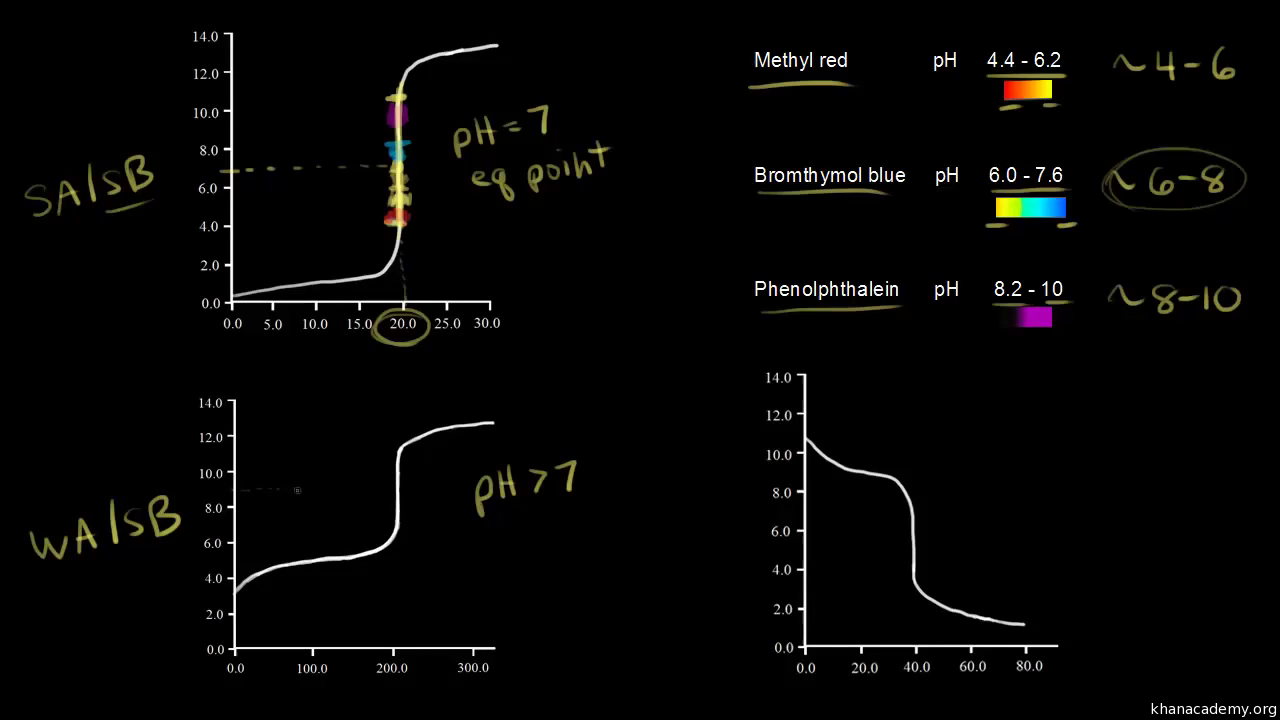
You might even see some green in there. Now why is bbt a good ph indicator for this titration? The choice of indicator will.
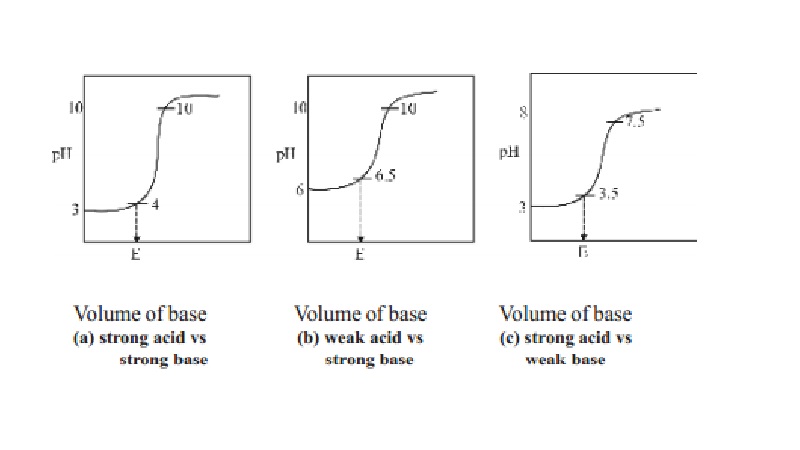
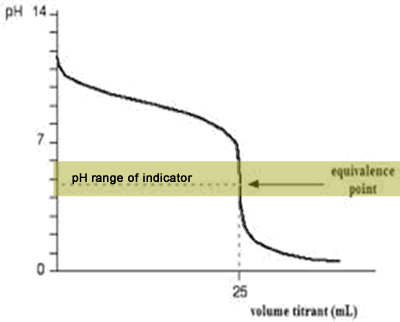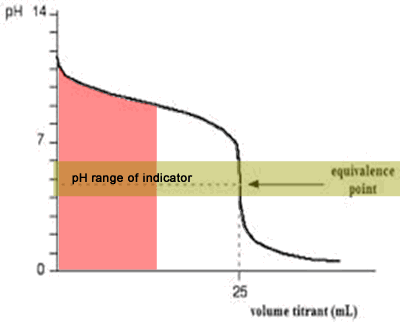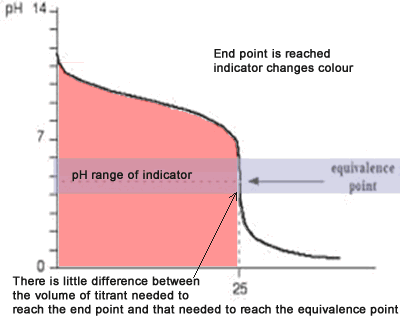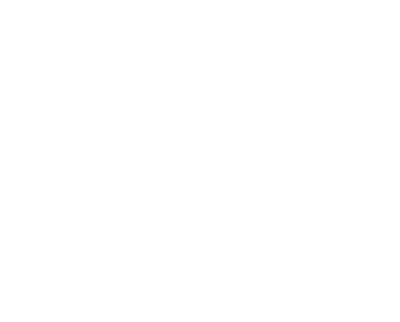
For example, in the titration of a strong acid. These compounds are added to the titrant and analyte during the titration process. Titrations involving strong acids and weak bases have an equivalence point in the acidic region of the ph scale.
Titration of weak acids and bases using bromothymol blue as an indication. How do you choose an indicator from pka? Use a table of indicator colour and ph range to choose an indicator which.
The appropriate indicators are selected for two titrations—a weak acid titrated with a strong base solution and a weak base solution titrated with a strong acid solution. The key point is that the colour change occurs when the equivalence is reached. When the blue color persists,.
Determine the ph of the solution at the equivalence point: The colour depends on the dominance of either the. For example, in the titration of a strong acid with a strong.

![18.3 Identify An Appropriate Indicator For A Titration [Hl Ib Chemistry] - Youtube](https://i.ytimg.com/vi/9Z5gWxxDcUQ/maxresdefault.jpg)