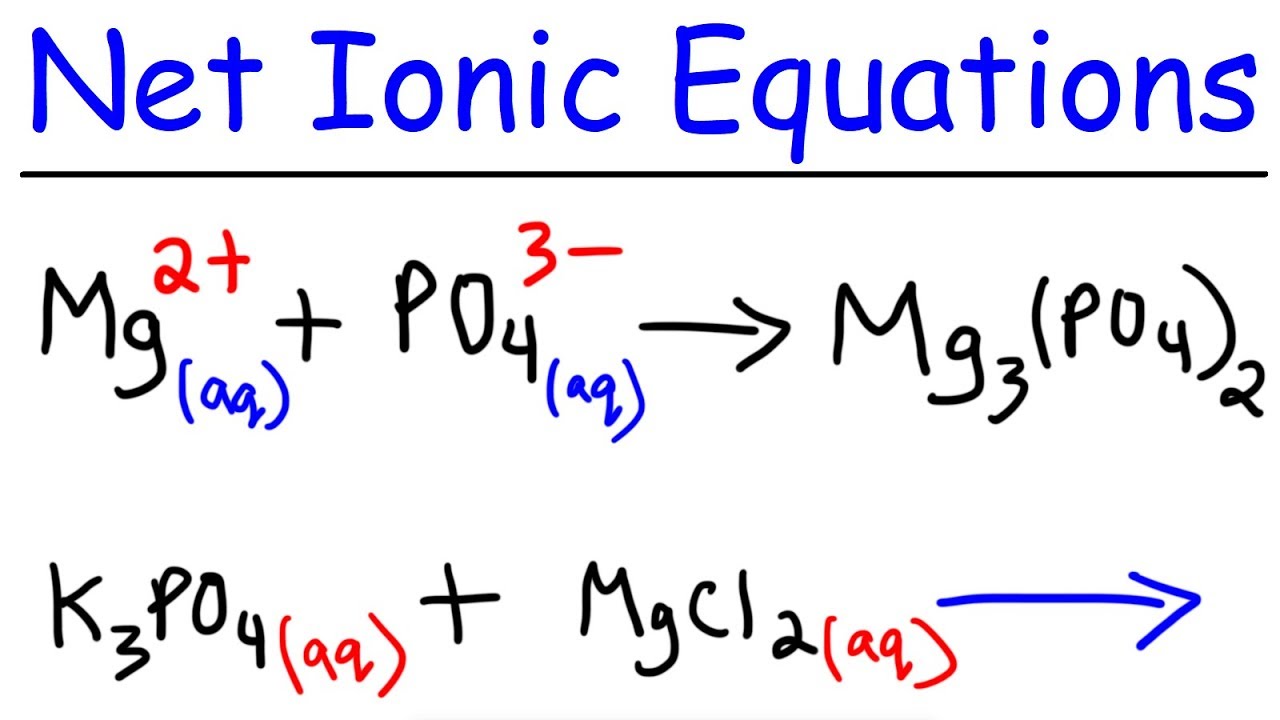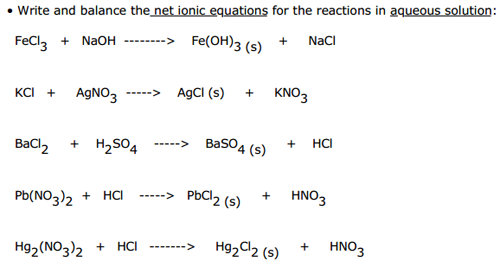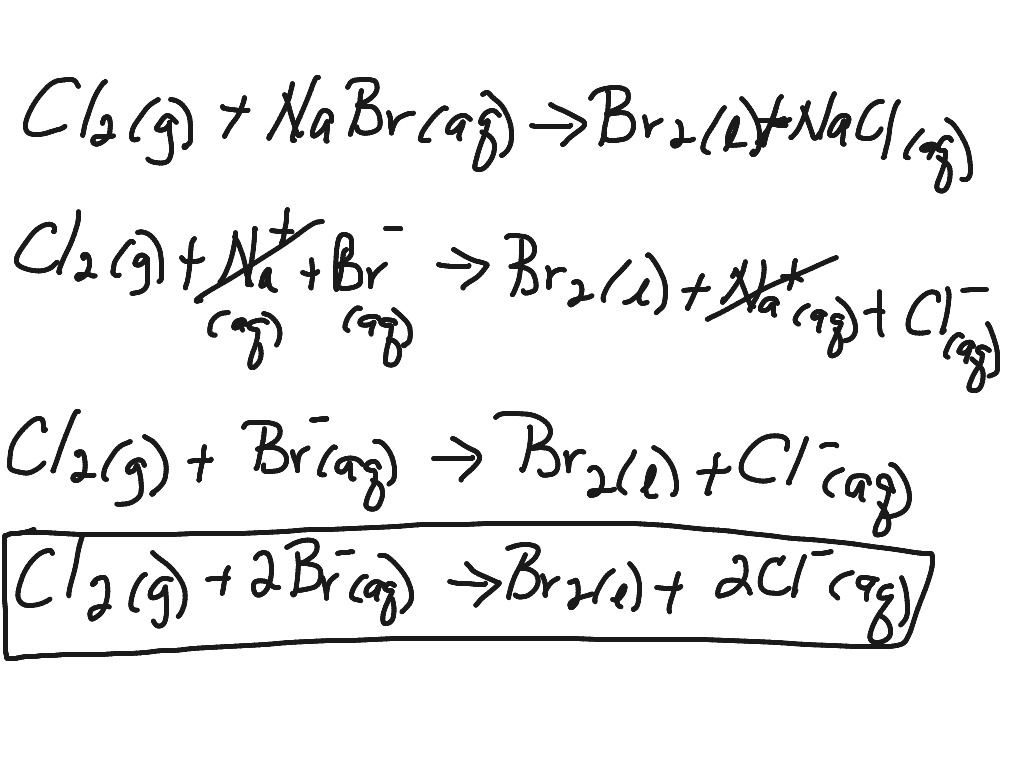Fantastic Info About How To Write Ionic Equations

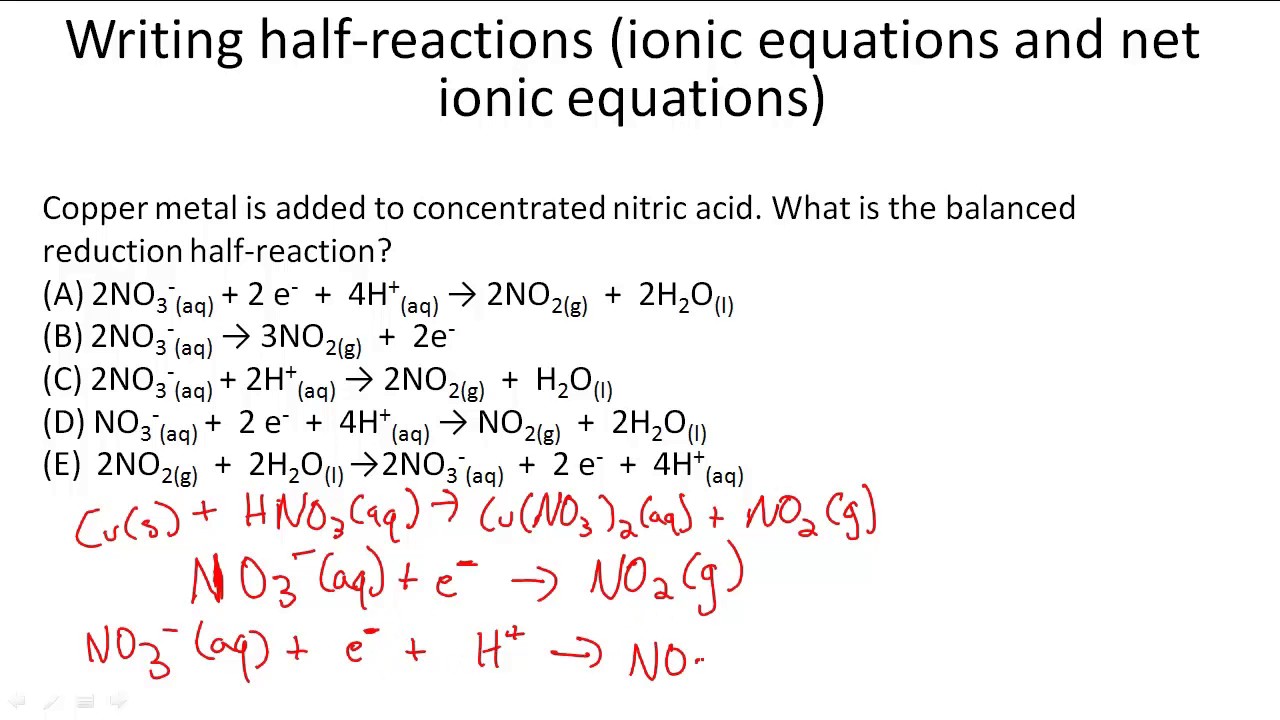
The general steps for writing net ionic equations are:
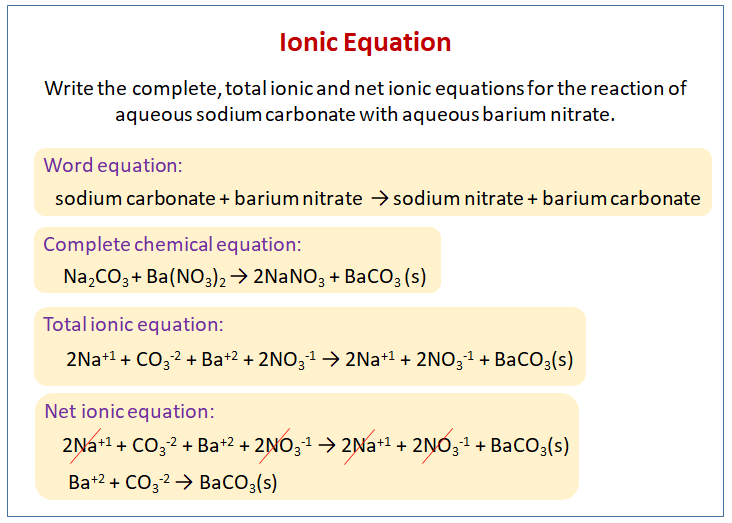
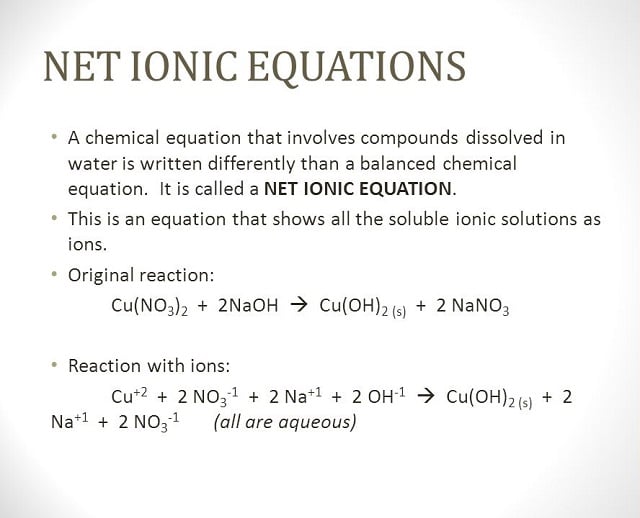
How to write ionic equations. Writing net ionic equations the first step to writing a net ionic equation is balancing the chemical equation present. This means you can ignore them when you write the ionic equation. Balancing and writing chemical equations teaching resources the science teacher.
How to write net ionic equations? Precipitation reactions involve two solutions reacting to form an insoluble product, the precipitate. Physical state identifiers are provided step 1:
In the input field, enter the required values or functions. It explains how to predict the products of double replacement reactions and acid ba. Let’s use the reaction between sodium chloride and silver nitrate as an.
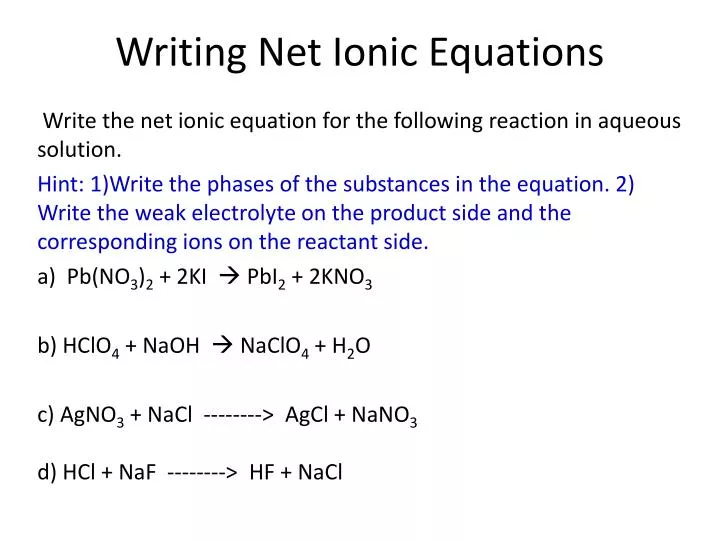
Writing a net ionic equation: How to write a net ionic equation there are three steps to. You only need to model how the solid silver chloride forms:
This chemistry video tutorial explains how to write net ionic equations. Look at the molecular equation and identify which species are in the aqueous state. Learn the basics about ionic equations.
Write the balanced molecular equation. Write the state (s, l, g, aq) for each substance. Split strong electrolytes into ions (the.